You Tube video explaining Glycolysis, Kreb Cycle and Electron Transport System
FILA Table of PBL 2
You Tube video explaining Glycolysis, Kreb Cycle and Electron Transport System
FILA Table of PBL 2
A letter to lecturer on the teaching method and the knowledge learned in biochemistry class.
Side note: A letter was sent to my respective lecturer Professor. Dr. Awang Sallehin. However, in case the email has not reach the recipient, attached is the file of my letter.
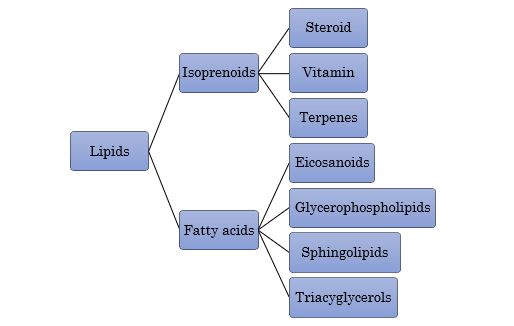
Generally, lipid is a group of compounds that is not soluble or partially soluble in water. Together with protein and carbohydrate, it is a major constituent in organisms and possess vital functions; energy stores, component of biological membranes and serves as signalling molecules. the classification of lipids is as illustrated in Figure 1.
Fatty acids are hydrocarbon structures formed by four (C4) or more carbons attached to carboxyl group. Short, medium, long and very long chain-fatty acids are classified based on carbon number which are C4-C10, C12-C14, C16-C18 and C20 respectively. C16 and C18 are predominant in plant and animal respectively. Additionally, fatty acids can also be classified based on the degree of saturation. The key feature of saturated chain are molecules are packed tightly in rigid and organized. For example, laurate, myristate and palmitate. Whilst, unsaturated chain is the opposite; ununiformly packed particles that can move. For instance, oleate, linoleate and palmitoleate.
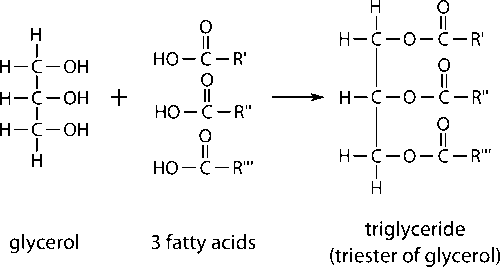
Triacylglycerols (TAG) is an ester derived from glycerol and three fatty acids as illustrated in Figure 2. Not only serves as energy storage, TAG or commonly known as body fat is use as body insulation in human and animal. Chemically, its melting temperature reflect their composition. As the chain length increase, molecular weight also increases resulting in increasing melting point. Besides, saturated chain has higher melting point than unsaturated chain.
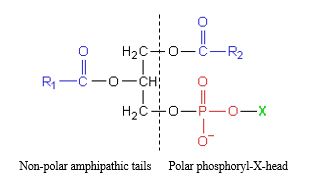
Glycerophospholipids are major component of biological membranes, i.e.: lipid bilayer. They are amphipathic molecules where glycerol and phosphate act as the polar end while hydrocarbon chains act as the nonpolar end.
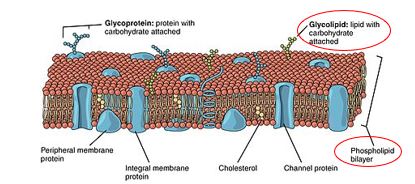
Sphingolipids is another type of phospholipid besides glycerophospholipids. It is also an amphipathic molecule and a major membrane component. The hydrophobic region consists of sphingoid long chain base (aliphatic chain with attached hydroxyl group) with fatty acid chain attached by amide bond at carbon 2. The hydrophilic region has phosphate groups, sugar residues and hydroxyl groups. Sphingomyelins, cerebrosides and gangliosides are the examples of sphingolipids.
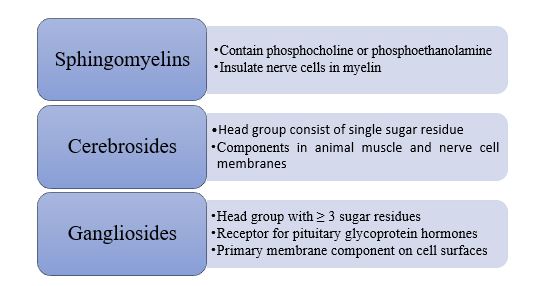
Steroids have a fused ring structure which do not resemble the other lipids. Despite that, they are grouped as lipid because of their hydrophobic property. All steroids have four linked carbon rings and majority has a short tail. Steroids that have the -OH group are classified as sterols. Cholesterol is one of the examples of steroid. It is common in animal which function as salt balancer, involve in metabolic functions and sexual functions.
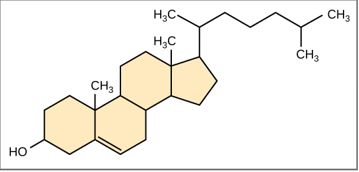
Terpenes are non-saponifiable lipids that is derived biosynthetically from units of isoprene. They are categorized based on the number of isoprene units; monoterpenes, sesquiterpenes, diterpenes, sesterterpenes and triterpenes for 2,3,4,5 and 6 isoprene units respectively. Isopentenyl pyrophosphate (IPP) and Dimethyl pyrophosphate (DMAPP) are the examples of terpenes. Terpenes are the major biosynthetic building blocks within living creature. Some terpenes are used to attract pollinating insects or providing defence mechanism by attracting animals, such as mites. In plants, they are common constituent of essential oils which employ wide range of natural flavour additives for food and as fragrance in perfumery.
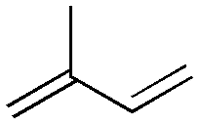
Eicosanoids are signalling molecules made by oxidation of arachidonic acid (or other polyunsaturated fatty acids that have 20 carbon units like arachidonic acid). Arachidonic acid is a polyunsaturated fatty acid and a type of omega-6 fatty acids. They can be either omega-6 fatty acids derived, or omega-3 fatty acids derived. The synthesized eicosanoids are not stored in body, instead they are directly used on site. Eicosanoids have complex control over bodily system such as inflammation and immunity. They also act as messenger in the central nervous system. They have roles towards the production of pain and fever, regulation of blood pressure, blood coagulation and reproduction. Eicosanoids are further classified under subfamilies; prostaglandins, prostacyclin, thromboxane and leukotrienes.
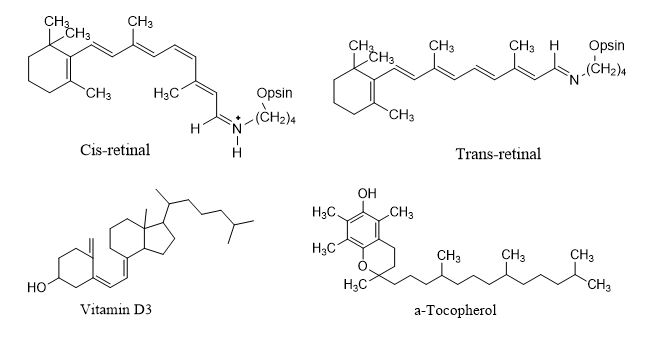
The four lipid soluble vitamins present in diet are vitamin A, D, E and K. Vitamin is a group of unsaturated organic compound consist of retinol, retinoic acid, provitamin A carotenoids and beta-carotene. Vitamin A plays an important role in the visual cycle of rod cell. Retinal (a vitamin A aldehyde) is the active molecules of this cycle. It forms an imine with an -NH2 group of the protein opsin to form visual pigment called rhodopsin. The primary chemical event of vision in rod cells is absorption of light by rhodopsin followed by isomerization of the 11-cis double bond to the 11-trans double bond. Next, vitamin D is a group of structurally related compounds that play a role in the regulation of calcium and phosphorus metabolism. Vitamin D3 is the most abundant form in the circulatory system. Vitamin E is a group of structurally related compounds which function as antioxidant. α-tocopherol is the most active compound of vitamin E. they trap HOO• and ROO• radicals formed as a result of oxidation by oxygen of unsaturated hydrocarbon chains in membrane phospholipids.

Compounds with empirical formula Cn(H2O)n are classified as carbohydrate. Carbohydrate is a biological macromolecule which functions involves energy storage, structural components and cellular recognition. Produced during photosynthesis through carbon dioxide fixation, it is also referred as hydrates of carbon because of the 1:2 ratio of hydrogen and oxygen atom respectively as they are in water. Monosaccharide, disaccharide and polysaccharide are the three major types of carbohydrate. Monosaccharides is the basic unit of carbohydrate followed by disaccharides with two to ten monosaccharide units whilst polysaccharide is the polymer of carbohydrates.
The hydrolysis of monosaccharides into smaller unit cannot be undergo under mild condition because it is the simplest form of carbohydrate. Monosaccharides compounds are classified based on the number of carbons. For instance, compound of C3(H2O)3 has three carbon atoms which classified as triose. Monosaccharides with aldehyde or ketone group are classified into aldoses and ketoses respectively. Aldoses with more than 2 carbon atoms and ketoses with more than three carbon atoms possess chiral carbon therefore have stereoisomer. The functional group of cyclized monosaccharides form hemiacetal with C-O-C bridge whilst the uncyclized form act as reducing agent. The derivatives of monosaccharides include sugar phosphate, deoxy acids, amino sugars and sugar alcohol.
Disaccharides is glycosides that form between two monosaccharides through glycosidic bond. Glycosidic bond is identified by the linkage from the anomeric carbon of a monosaccharide compound to the -OR group of another monosaccharide compound. For instance, both monosaccharides unit of maltose consist of D-glucose monomers in α(1→4) linkage and occasional branch points with a α(1→6) linkage. Maltose, cellobiose, sucrose and lactose are some example of disaccharides.
Polysaccharide is further classified into homopolysaccharide and heteropolysaccharide. Homopolysaccharide has only one type of monomer whilst heteropolysaccharide possess many types of monomers. Polysaccharides have three key functions which are energy storage, structural components and cellular recognition.
The energy stored in plant and animal that are termed as starch and glycogen respectively are polysaccharides compounds. Starch is further classified into amylose and amylopectin based on their chemical structure. Generally, amylose is a linear polymer of α(1→4) linked glucose residue whilst amylopectin is a branched polymer of α(1→4) linked glucose residue with a α(1→6) linked branch. Glycogen has quite similar configuration as amylopectin where the only difference is branches of glycogen are formed every 8-12 glucose whilst branches of amylopectin are formed every 24-30 glucose. Storage carbohydrate highlighting starch are the major energy source for human diet.
Some polysaccharides function as structural component which is chitin, cellulose and glucoaminoglycans. Classified as homopolymer, chitin is the main component of cell wall of fungi and exoskeletons of arthropods. Cellulose is a homopolymer which function as primary component of plant cell wall and green algae. Lastly, glucoaminoglycan is a long, unbranched polysaccharides made of repeating disaccharides units. Unlike storage carbohydrate, cell wall polysaccharide in plant is not digested. However, it is a major component of dietary fibre that is important in normalising bowel movement.
Glycoprotein is a polysaccharide compound and an important integral membrane protein with vital role in cell-cell interaction. Generally, glycoprotein is further classified into two types, O- linked glycoprotein and N- linked glycoprotein based on their chemical structure. Besides cell-cell interaction, glycoprotein is important in embryonic development, cell adhesion, immune function and pathogenic configuration.
In summary, the three major types of carbohydrates are monosaccharides, disaccharides and polysaccharides. Carbohydrate has important function in daily life such as storage carbohydrates are the major energy source for human diet, cell wall polysaccharides are vital as dietary fibre and lastly, informational carbohydrate function in cellular recognition and maintain body’s health.
Previous days ago my friends and I (in a group of 4) had finished a You Tube video explaining interactions between H2O molecules and salt (NaCl) when dissolved in water. If you are interested, the video is available above and feel free to watch it.
The post-activity of this task is to do a self-reflection about the video, discussing about the bonds within the water molecules and hydrogen bond interactions with other molecules like salt (NaCl). The good thing about this task is self-learning. Via self-learning, I can do extra reading, understand deeper about the topic and most importantly, felt satisfied of my own effort (even though it is a pretty simple interaction). Below is roughly what I understand about how salt is dissolved in water.
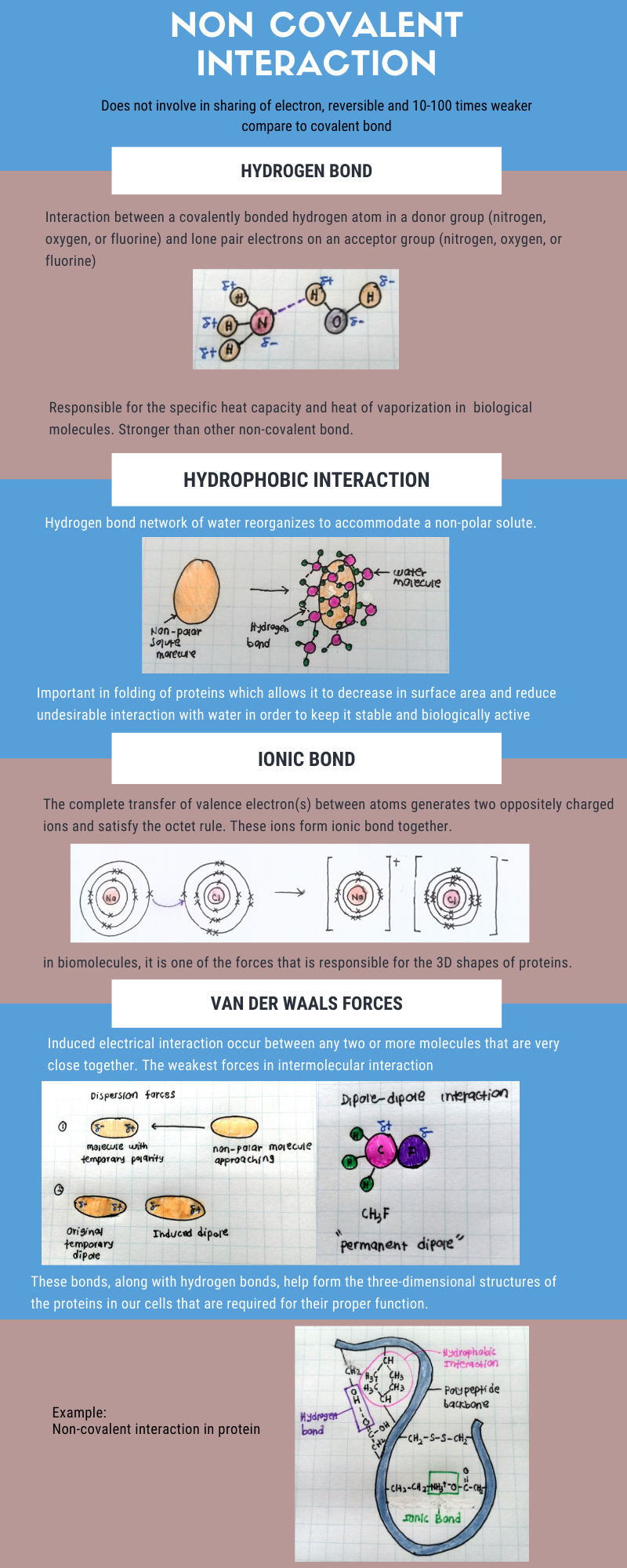
Basically, water is a polar molecule because of the uneven charge distribution. Since that oxygen is more electronegative than hydrogen, so oxygen atom is partially negative and hydrogen atom is partially positive. Sodium chloride or generally known as table salt is an ionic compound consist of sodium ion (an anion or a positively charged ion) and chloride ion (a cation or a negatively charged ion). When salt is dissolved in water, dissociation of salt occurs and water molecules are responsible for it. The partially negative side of the water is attracted to the chloride ion. Same things goes to sodium ion where the partially positive side of the water is attracted to sodium ion. The molecular-ion attraction on both side break the ionic bond of sodium chloride and pull the anion and cation apart. At this point, dissociation occur or in other term salt is dissolved in water.
If you ever thought of why not water molecules is pulled apart instead of salt, it is because water molecules has covalent bond. In a covalent bond, the electrons are shared between atoms. As oxygen is more electronegative than hydrogen atom, it attract electrons stronger compare to hydrogen. The unequal sharing of electrons result in polarity of water molecule. The fact that covalent bond is much stronger than ionic bond let salt dissociate instead.
Lastly, hydrogen bond is an interaction between a covalently bonded hydrogen atom in a donor group and a pair of non-covalent electrons on an acceptor group. The ability of a group to act as a hydrogen bond donor depends on its electronegativity. Generally, only Oxygen, Nitrogen and Fluorine atom have sufficient electronegativity to act as hydrogen bond donor. Besides, a hydrogen bond acceptor must possess a lone pair for the formation of a hydrogen bond. Water able to form hydrogen bonding within themselves because of the presence of oxygen atom. Sodium and chloride ion does not form hydrogen bond with water molecules because of lacking in electronegativity. They are bound to water by electrostatic force as water is a polar molecules.